| Identification | Back Directory | [Name]
NXL 104 | [CAS]
1192491-61-4 | [Synonyms]
NXL 104
Avabatan
AVE-1330A
Avibactam-001-Na
Avibactam (sodium)
AvibactaM SodiuM Sal
AvibactaM SodiuM Salt
NXL-104;NXL 104;NXL104
AvibactaM SodiuM NXL 104
SODIUM;[(2S,5R)-2-CARBAMOYL-7-OXO-1,6-DIAZABICYCLO[3.2.1]OCTAN-6-YL] SULFATE
sodium (1R,2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl sulfate
(2S,5R)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide 7-oxo-6-(sulfoxy)monosodium salt
(2S,5R)-7-Oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide monosodium salt
Sulfuric Acid Mono[(1R,2S,5R)-2-(aMinocarbonyl)-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl] Ester SodiuM Salt
Sulfuric acid, mono[(1R,2S,5R)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl] ester, sodium salt (1:1) | [Molecular Formula]
C7H10N3NaO6S | [MDL Number]
MFCD28900719 | [MOL File]
1192491-61-4.mol | [Molecular Weight]
287.226 |
| Chemical Properties | Back Directory | [Melting point ]
>208°C (dec.) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly, Heated, Sonicated), Water (Slightly, Sonicated) | [form ]
Solid | [color ]
White to Off-White | [Water Solubility ]
H2O: 2mg/mL, clear | [InChI]
InChI=1/C7H11N3O6S.Na.H/c8-6(11)5-2-1-4-3-9(5)7(12)10(4)16-17(13,14)15;;/h4-5H,1-3H2,(H2,8,11)(H,13,14,15);;/t4-,5+;;/s3 | [InChIKey]
JKMUNBAKQJMVKG-NLZGLQCHNA-N | [SMILES]
C([C@@H]1CC[C@H]2N(OS(O)(=O)=O)C(=O)[N@@]1C2)(=O)N.[NaH] |&1:1,4,13,r| |
| Hazard Information | Back Directory | [Description]
Avibactam sodium (NXL-104, 1192491-61-4), a diazabicyclooctanone compound, is currently the most favoured novel β-lactamase inhibitor. Compared with the three marketed β-lactamase inhibitors, NXL-104 has a long duration of action and reversible covalent binding to the enzyme, and does not induce β-lactamase production.
| [Characteristics]
Avibactam sodium is a novel beta-lactamase inhibitor. In its structure, Na+ readily forms coordination bonds with water molecules. In addition, there are several O atoms as hydrogen bond acceptors and -NH2 as hydrogen bond donors. As a result, avibactam sodium crystallises easily into its hydrated form[1]. The structure is shown below:
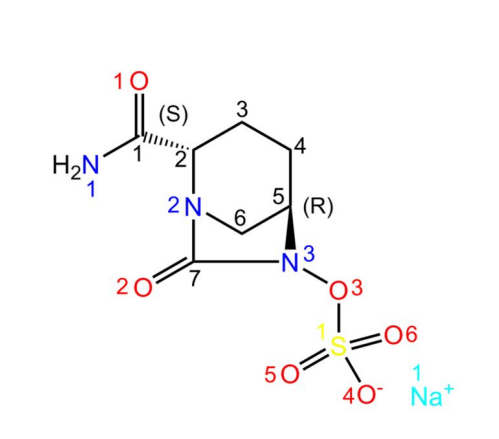 | [History]
Avibactam sodium and its free acid, avibactam, are diazabicyclooctane (DBO)–based, non-β-lactam β-lactamase inhibitors (BLIs) that are used to treat multi-drug–resistant Gram-negative bacterial pathogens. The compounds were jointly developed by Actavis Generics (now part of Teva Pharmaceutical Industries) and AstraZeneca.
In 2015, avibactam sodium, in combination with ceftazidime, a cephalosporin antibiotic, was approved by the US Food and Drug Administration. The dual-drug’s target was complex urinary tract and intra-abdominal infections caused by Gram-negative, often hospital-acquired, bacteria.
In 2018, in the continuing quest to combat multi-drug–resistant bacteria, Eric M. Gordon, Matthew A. J. Duncton, and Mark A. Gallop at Arixa Pharmaceuticals (Palo Alto, CA) prepared analogues of avibactam that are more orally available than the free acid or its salt. | [Uses]
Avibactam (NXL104) is a novel β-lactamase inhibitor with a non-lactam structural scaffold. NXL104 irreversibly inhibits lactamase from Mycobacterium tuberculosis. | [Definition]
ChEBI: An organic sodium salt that is the monosodium salt of avibactam. Used in combination with ceftazidime pentahydrate for the treatment of complicated urinary tract infections including pyelonephritis. | [Biological Activity]
Avibactam is a β-lactamase inhibitor (IC50s = 8, 80, and 38 nM for TEM-1, P99, and KPC-2 β-lactamases, respectively). It restores the antimicrobial activity of ceftazidime , ceftriaxone , imipenem , and piperacillin against antibiotic-resistant Enterobacteriaceae in vitro (MIC90 reduction 4-1,024-fold across 190 E. coli and K. pneumoniae clinical isolates). Formulations containing avibactam have been used to treat carbapenem-resistant Enterobacteriaceae infections. | [Synthesis]
A solution of sodium 2-ethyl hexanoate (475.0 g, 2.850 mol) in ethanol (2.00 L) was added to a solution of tetrabutylammonium [(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl] sulfate 13 (723.0 g) in ethanol (2.50 l) and water (50 mL) over approximately 1 h, and the temperature was maintained at rt. The reaction mixture was held for 2 h. The product was filtered, washed with ethanol (2 × 2.0 L), and dried to yield a white crystalline solid 1 (395.0 g, 96.2%), mp 259.1–262.4 °C (decomposition); [α]D20 = ?46.40 (c = 0.79, MeOH/H2O = 1/1); 1H NMR (500 MHz, D2O) δ 4.15 (dd, J = 5.8, 2.8 Hz, 1H), 4.01 (d, J = 7.5 Hz, 1H), 3.28 (d, J = 12.2 Hz, 1H), 3.06 (d, J = 12.2 Hz, 1H), 2.23–2.09 (m, 1H), 2.06–1.96 (m, 1H), 1.94–1.82 (m, 1H), 1.81–1.69 (m, 1H). 13C NMR (126 MHz, D2O) δ 174.72 (s), 169.53 (s), 60.43 (s), 59.93 (s), 47.33 (s), 20.03 (s), 18.31 (s). IR (cm–1): 3459, 1749, 1675, 1361, 1270, 1013, 857, 768. MS (ESI) m/z: 279.0 [M + H]+.
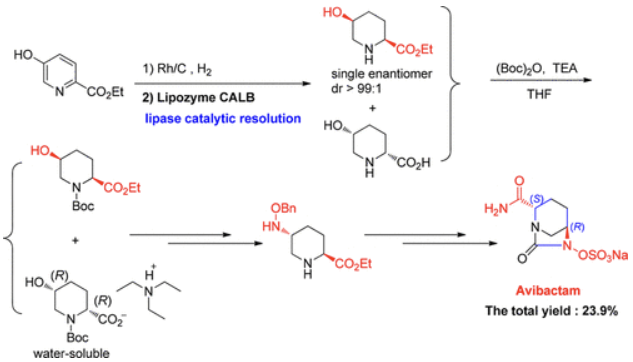 | [IC 50]
CTX-M-15: 5 nM; TEM-1: 8 nM | [References]
[1] STACHYRATHéRèSE. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor.[J]. Antimicrobial Agents and Chemotherapy, 2010: 5132-5138. DOI:10.1128/AAC.00568-10.
[2] BONNEFOYALAIN. In vitro activity of AVE1330A, an innovative broad-spectrum non-beta-lactam beta-lactamase inhibitor.[J]. Journal of Antimicrobial Chemotherapy, 2004, 54 2: 410-417. DOI:10.1093/jac/dkh358.
[3] LAGACé-WIENSP R S. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases.[J]. Antimicrobial Agents and Chemotherapy, 2011, 55 5: 2434-2437. DOI:10.1128/AAC.01722-10.
[4] KINGMADELINE. Multicenter Study of Outcomes with Ceftazidime-Avibactam in Patients with Carbapenem-Resistant Enterobacteriaceae Infections.[J]. Antimicrobial Agents and Chemotherapy, 2017, 61 7. DOI:10.1128/AAC.00449-17.
[5] ZHIYONG DING. Understanding the Role of Water in Different Solid Forms of Avibactam Sodium and Its Affecting Mechanism[J]. Crystal Growth & Design, 2020, 20 2: 1150-1161. DOI:10.1021/acs.cgd.9b01459. |
|
|