| Identification | Back Directory | [Name]
Duloxetine | [CAS]
116539-59-4 | [Synonyms]
DULOXETIN
DULOXETINE
DULOXETINE-D3
(S)-DULOXETINE
DULOXETINE HCI
Duloxetine Hcl(S)
Duloxetine & Intermediates
methyl-[(3S)-3-(1-naphthyloxy)-3-(2-thienyl)propyl]amine
N-Methyl-gama-(1-naphthalenyloxy)-2-thiophenepropanamine
Methyl[(3S)-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propyl]aMine
(3S)-N-methyl-3-naphthalen-1-yloxy-3-thiophen-2-yl-propan-1-amine
(3R)-N-methyl-3-(naphthalen-1-yloxy)-3-thiophen-2-ylpropan-1-amine
(S)-N-Methyl-3-(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-1-aMine | [EINECS(EC#)]
601-438-0 | [Molecular Formula]
C18H19NOS | [MDL Number]
MFCD06801358 | [MOL File]
116539-59-4.mol | [Molecular Weight]
297.41 |
| Chemical Properties | Back Directory | [Boiling point ]
466.2±40.0 °C(Predicted) | [density ]
1.158±0.06 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
Soluble in DMSO | [pka]
10.02±0.10(Predicted) | [BCS Class]
2 | [InChI]
InChI=1/C18H19NOS.ClH/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16;/h2-10,13,17,19H,11-12H2,1H3;1H/t17-;/s3 | [InChIKey]
BFFSMCNJSOPUAY-VOPAOICTNA-N | [SMILES]
C1(=CC=CS1)[C@H](CCNC)OC1=CC=CC2=CC=CC=C12.Cl |&1:5,r| | [EPA Substance Registry System]
2-Thiophenepropanamine, N-methyl-?-(1-naphthalenyloxy)-, (?S)- (116539-59-4) |
| Hazard Information | Back Directory | [Uses]
Antidepressant. | [Definition]
ChEBI: (S)-duloxetine is a duloxetine. It is an enantiomer of a (R)-duloxetine. | [Brand name]
Cymbalta (Lilly). | [General Description]
Duloxetine (Cymbalta) is a newer antidepressant. It islargely like venlafaxine, which is an SNERI (selective norepinephrinereuptake inhibitor). | [Pharmacokinetics]
Duloxetine appears to be fairly well absorbed after oral doses, with peak plasma levels in 6 to 10 hours and
linear pharmacokinetics. The drug is extensively metabolized in the liver to active
metabolites, with 72% of an oral dose primarily excreted in the urine as conjugated metabolites and up to
15% appearing in the feces.
N-demethylation to an active metabolite (CYP2D6) and hydroxylation of the naphthyl ring (CYP1A2) at either
the 4-, 5-, or 6-positions are the main metabolic pathways for duloxetine. Its metabolites are primarily
excreted into the urine as glucuronide, sulfate, and O-methylated conjugation products. The
major metabolites found in plasma also were found in the urine. Preclinical data for 4-hydroxyduloxetine
suggests it has a similar pharmacological profile to duloxetine, with selective inhibition of SERT but less
activity at the NET. | [Clinical Use]
Duloxetine has been approved for the treatment of depression and diabetic peripheral
neuropathic pain. It is another analogue in the line of fluoxetine-based products from Lilly, in which the phenyl and phenoxy groups of fluoxetine have been respectively replaced with the benzene isostere,
thiophene, and a
naphthyloxy group (previously described under fluoxetine). Duloxetine exhibits dual inhibition with high
affinity for the SERTs and NETs, with a five times preferential inhibition of the SERT. Duloxetine
appears to be a more potent in vitro blocker of SERTs and NETs than venlafaxine. In humans, duloxetine has
a low affinity for the other neuroreceptors, suggesting low incidence of unwanted adverse effects. | [Synthesis]
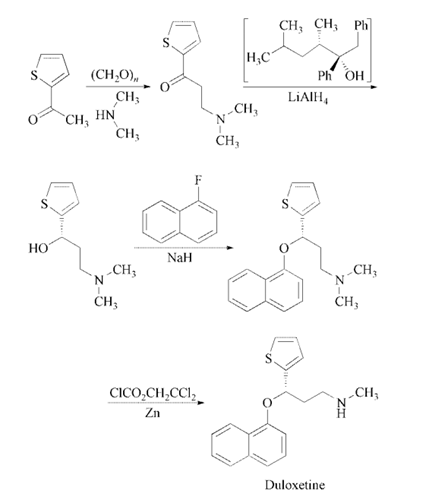
Reaction of 2-acetylthiophene
with paraformaldehyde and dimethylamine in
ethanol gives 3-(dimethylamino)-1-(2-thienyl)-
1-propanone, which is enantioselectively reduced
with a 2:1 complex of (2R,3S)-4-(dimethylamino)-
3-methyl-1,2-diphenyl-2-butanol
and LiAlH4 in toluene to yield (S)-3-(dimethylamino)-
1-(2-thienyl)-1-propanol. The
condensation of (S)-3-(dimethylamino)-1-(2-
thienyl)-1-propanol with 1-fluoronaphthalene
catalyzed by NaH in DMSO affords the corresponding
naphthyl ether (S)-N,N-dimethyl-3-
(naphthalen-1-yloxy)-3-(thiophen-2-yl)propan-
1-amine, which is finally monodemethylated
with 2,2,2-trichloroethyl chloroformate and zinc
in toluene and treated with oxalic acid .
 | [Drug interactions]
Potentially hazardous interactions with other drugs
Antibacterials: metabolism inhibited by ciprofloxacin
- avoid.
Anticoagulants: possibly increased risk of bleeding
with dabigatran.
Other CNS medication: enhanced effect.
Antidepressants: avoid with MAOIs, moclobemide,
St John’s wort, tryptophan, venlaflaxine, amitriptyline,
clomipramine and SSRIs due to increased risk of
serotonin syndrome; increased risk of side effects
with tricyclic antidepressants; fluvoxamine decreases
the clearance of duloxetine by 77% - avoid; possible
increased risk of convulsions with vortioxetine.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Dapoxetine: avoid concomitant use.
Methylthioninium: risk of CNS toxicity - avoid if
possible. | [Metabolism]
Duloxetine is extensively metabolised and the metabolites
are excreted principally in urine. Both cytochromes
P450-2D6 and 1A2 catalyse the formation of the two major
metabolites, glucuronide conjugate of 4-hydroxy duloxetine
and sulphate conjugate of 5-hydroxy, 6-methoxy duloxetine.
Based upon in vitro studies, the circulating metabolites of
duloxetine are considered pharmacologically inactive |
|
|