| Identification | Back Directory | [Name]
1,2-O-Isopropylidene-a-L-xylofuranose | [CAS]
114861-22-2 | [Synonyms]
SOTA-002
1,2-O-isopropylidene-α-L-xylofuranose
1,2-O-Isopropylidene-a-L-xylofuranose
1,2-O-Isopropylidene-alpha-L-xylofuranose
a-L-Xylofuranose,1,2-O-(1-methylethylidene)-
α-L-Xylofuranose, 1,2-O-(1-Methylethylidene)-
(3AS,5S,6R,6AS)-5-(HYDROXYMETHYL)-2,2-DIMETHYL-3A,5,6,6A-TETRAHYDROFURO[2,3-D][1,3]DIOXOL-6-OL | [Molecular Formula]
C8H14O5 | [MDL Number]
MFCD00010529 | [MOL File]
114861-22-2.mol | [Molecular Weight]
190.19 |
| Chemical Properties | Back Directory | [Boiling point ]
333.0±37.0 °C(Predicted) | [density ]
1.267±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [pka]
13.23±0.60(Predicted) |
| Hazard Information | Back Directory | [Uses]
1,2-O-Isopropylidene-a-L-xylofuranose can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly in laboratory research and development processes and chemical production processes. | [Synthesis]
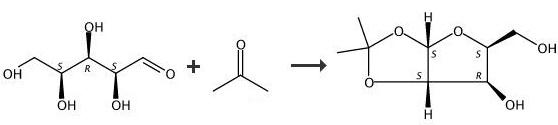
((3aS,5R,6S,6aS)-6-Hydroxy-2,2-dimethyltetrahydrofuro[3,2-d][1,3]dioxol-5-yl)(morpholino)methanone (134). Step 1) (3aS,5S,6R,6aS)-5-(Hydroxymethyl)-2,2-dimethyltetrahydrofuro[3,2-d][1,3]dioxol-6-ol. To a suspension of L-(-)-xylose (133, 19.15 g, 127.5 mmol) and MgSO4 (30.72 g, 255.0 mmol) in acetone (190 mL) was added conc. H2SO4 (1.9 mL) at room temperature. After 12 h, the reaction mixture (all L-(-)-xylose had been consumed) was filtered and the collected solids were washed with acetone (twice, 20 mL per wash). The stirring yellow filtrate was neutralized with NH4OH solution to pH ~ 9. The suspended solids were removed by filtration. The filtrate was concentrated to afford crude bis-acetonide intermediate as yellow oil. The yellow oil was suspended in water (5 mL), and then the pH was adjusted from 9 to 2 with 1 N HCl in water solution. The reaction mixture was stirred for 12 h at room temperature. The resulting mixture was neutralized by the addition of 25% (w/w) K3PO4 in water until pH ~ 7. The mixture was extracted with EtOAc. The organic layer was dried over MgSO4 filtered, and concentrated in vacuo. The crude product was purified by silica gel column chromatography. Yield: (12.63 g, 52%) as yellow oil. 1H NMR (400 MHz, CD3OD) δ 5.88 (d, J = 4.0 Hz, 1H), 4.47 (d, J 4.0 Hz, 1H), 4.18-4.14 (m, 1H), 4.1 1 (d, J = 2.8 Hz, 1H), 3.83-3.71 (m, 2H), 1.45 (s, 3H), 1.29 (s, 3H). |
|
|