| Identification | Back Directory | [Name]
Vitamin A | [CAS]
11103-57-4 | [Synonyms]
ROIDEX
Aquasol A
Vitamin A
Vitamin A Retinol
Water-soluble vitamin A
3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)-nona-2,4,6,8-tetraen-1-ol | [EINECS(EC#)]
234-328-2 | [Molecular Formula]
C20H30O | [MDL Number]
MFCD00001552 | [MOL File]
11103-57-4.mol | [Molecular Weight]
286.46 |
| Chemical Properties | Back Directory | [Appearance]
Retinol acetate: pale-yellow crystals (mp: about 60 °C). Once melted retinol acetate tends to yield a supercooled melt. Retinol propionate: reddish-brown oily liquid. Retinol palmitate: a fat-like, light yellow solid or a yellow oily liquid, if melted (mp: about 26 °C). | [storage temp. ]
-20°C | [solubility ]
All retinol esters are practically insoluble in water, soluble or partly soluble in anhydrous ethanol and miscible with organic solvents. Vitamin A and its esters are very sensitive to the action of air, oxidising agents, acids, light and heat. Carry out the assay and all tests as rapidly as possible, avoiding exposure to actinic light and air, oxidising agents, oxidation catalysts (e.g. copper , iron), acids and heat; use freshly prepared solutions. | [Uses]
vitamin A can act as a keratinization regulator, helping to improve the skin’s texture, firmness, and smoothness. Vitamin A esters, once in the skin, convert to retinoic acid and provide anti-aging benefits. Vitamin A is believed to be essential for the generation and function of skin cells. Continued vitamin A deficiency shows a degeneration of dermal tissue, and the skin becomes thick and dry. Surface application of vitamin A helps prevent skin dryness and scaliness, keeping the skin healthy, clear, and infection resistant. Its skin regeneration properties appear enhanced when combined with vitamin e. Vitamin A is a major constituent of such oils as cod liver and shark, and many fish and vegetable oils. See also retinol; retinoic acid; retinylpalmitate. | [EPA Substance Registry System]
Vitamin A(11103-57-4) |
| Hazard Information | Back Directory | [General Description]
Yellow crystals or orange solid. Practically water insoluble. | [Air & Water Reactions]
Practically water insoluble. | [Reactivity Profile]
Vitamin A is light sensitive. Also air sensitive and heat sensitive. Reacts with strong oxidizing agents. Also can react with acids . Can react exothermically with reducing agents to release hydrogen gas. | [Fire Hazard]
Flash point data for Vitamin A are not available. Vitamin A is probably combustible. | [Chemical Properties]
Retinol acetate: pale-yellow crystals (mp: about 60 °C). Once melted retinol acetate tends to yield a supercooled melt. Retinol propionate: reddish-brown oily liquid. Retinol palmitate: a fat-like, light yellow solid or a yellow oily liquid, if melted (mp: about 26 °C). | [History]
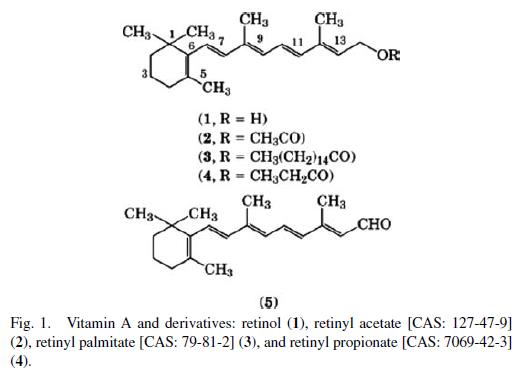
The structure of vitamin A and some of the important derivatives are shown in Figure 1. The parent structure is all-trans-retinol [CAS: 68-26-8] and its IUPAC name is (all-E)-3,7-dimethyl-9-(2,6,6- trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraen-1-ol. The numbering system for vitamin A derivatives parallels the system used for the carotenoids. In older literature, vitamin A compounds are named as derivatives of trimethyl cyclohexene and the side chain is named as a substituent. For retinoic acid derivatives, the carboxyl group is denoted as C-1 and the trimethyl cyclohexane ring as a substituent on C-9. The structures of vitamin A and β-carotene were elucidated by Karrer in 1930 and several derivatives of the vitamin were prepared by this group. In 1935, Wald isolated a substance found in the visual pigments of the eye and was able to show that this material was identical with Karrer’s retinaldehyde.
 | [Definition]
ChEBI: All-trans-retinol is a retinol in which all four exocyclic double bonds have E- (trans-) geometry. It has a role as a human metabolite, a mouse metabolite and a plant metabolite. It is a retinol and a vitamin A. | [Clinical Use]
Vitamin A is used in the treatment of known or suspected vitamin A deficiency. Of course, vaccination is of prime importance; however, in underdeveloped countries where the rate of vaccination is low, vitamin A supplementation is a beneficial alternative.Retinoate analogs have been developed for use in the treatment of dermatological conditions such as psoriasis and acne. | [storage]
Store at -20°C |
|
|