| Identification | Back Directory | [Name]
Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl] | [CAS]
1100598-32-0 | [Synonyms]
CS-977
Tepotinib
Veledimex
MSC2156119
EMD-1214063
EMD1214063,Tepotinib
epotinib(EMD 1214063)
EMD 1214063; EMD1214063
Tepotinib (EMD 1214063)
3-(1-(3-(5-((1-Methylpiperidin-4-yl)methoxy)pyrimidin-2-yl)benzyl)-6-oxo-1,6-dihydropyridazin-
3-(1-(3-(5-((1-METHYLPIPERIDIN-4-YL)METHOXY)PYRIMIDIN-2-YL)BENZYL)-1,6-DIHYDRO-6-OXOPYRIDAZIN-3-YL)B
3-[1-[[3-[5-[(1-methylpiperidin-4-yl)methoxy]pyrimidin-2-yl]phenyl]methyl]-6-oxopyridazin-3-yl]benzonitrile
3-(1-(3-(5-((1-Methylpiperidin-4-yl)Methoxy)pyriMidin-2-yl)benzyl)-6-oxo-1,6-dihydropyridazin-3-yl)benzonitrile
3-[1,6-Dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]benzonitrile
Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl]
Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl] USP/EP/BP
EMD-1214063
3-[1,6-Dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]benzonitrile
3-[1,6-Dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]benzonitrile EMD-1214063 | [Molecular Formula]
C29H28N6O2 | [MDL Number]
MFCD18452823 | [MOL File]
1100598-32-0.mol | [Molecular Weight]
492.572 |
| Chemical Properties | Back Directory | [Boiling point ]
626.5±65.0 °C(Predicted) | [density ]
1.25 | [storage temp. ]
Store at -20°C | [form ]
Solid | [pka]
8.93±0.10(Predicted) | [Water Solubility ]
≥ 4.93 mg/mL in DMSO, <2.52 mg/mL in EtOH, <2.56 mg/mL in Water | [InChIKey]
AHYMHWXQRWRBKT-UHFFFAOYSA-N | [SMILES]
C(#N)C1=CC=CC(C2=NN(CC3=CC=CC(C4=NC=C(OCC5CCN(C)CC5)C=N4)=C3)C(=O)C=C2)=C1 |
| Hazard Information | Back Directory | [Description]
Tepotinib is a highly selective inhibitor against MET. In xenograft models, acquired resistance to EGFR TKIs via secondary EGFR T790 M mutations can be overcome with tepotinib treatment. | [Uses]
EMD 1214063 is a novel ATP-comptetitive inhibitor of the MET hepatocyte growth factor receptor and a novel kinase inhibitor and a therapeutic agent for neuroblastoma. Potent c-MET inhibitor. | [Brand name]
Tepmetko | [General Description]
Class: receptor tyrosine kinase;
Treatment: NSCLC with METex14;
Other name: EMD-1214063;
Oral bioavailability = 72%;
Elimination half-life = 32 h;
Protein binding = 94% | [Mechanism of action]
Tepotinib is a Kinase Inhibitor. The mechanism of action of tepotinib is as a Mesenchymal Epithelial Transition Inhibitor, and P-Glycoprotein Inhibitor. | [Pharmacology]
Tepotinib is a highly-selective inhibitor of MET kinase activity, with an average IC50
of approximately 1.7 nmol/L. It has a moderate duration of action
necessitating once-daily administration. Tepotinib has been associated
with the development of interstitial lung disease (ILD)/pneumonitis,
which can sometimes be fatal. Patients should be monitored closely for
signs of new or worsening respiratory symptoms (e.g. dyspnea, cough),
and treatment with tepotinib should be immediately withheld if
ILD/pneumonitis is suspected. If no other potential causes of
ILD/pneumonitis are identified, therapy with tepotinib should be
suspended indefinitely. | [Clinical Use]
Tepotinib is currently being evaluated in combination with EGFR TKI gefitinib and also a separate trial in NSCLC patients with MET exon 14 skipping mutation and MET amplification. | [Side effects]
- anxiety
- chest pain or tightness
- difficult or labored breathing
- dizziness or lightheadedness
- fast heartbeat
- fever or chills
- general feeling of discomfort or illness
- muscle or bone pain
| [Synthesis]
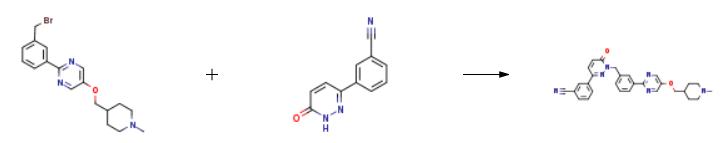
The synthesis of Tepotinib is as follows:
Acetonitrile (700 ml) was added to the reaction vessel, and the compound represented by formula 7a (37.63 g, 0.1 mol), the compound represented by formula 8 (23.66 g, 0.12 mol), and potassium carbonate (34.55 g, 0.25 mol) were added under stirring.and tetrabutylammonium bromide (1.93g, 0.006mol), the reaction system was stirred and heated to reflux, and the reaction was stirred at reflux for 12h. After the reaction was completed, the temperature was cooled to room temperature, the reaction solution was concentrated under reduced pressure to dryness, and ethanol (360ml) was added. ), stir to dissolve, slowly add 1.5 mol/l hydrochloric acid aqueous solution dropwise, adjust the pH value to 1-2, cool down to 5-7 °C, stir and crystallize for 6 h, filter, and vacuum dry the solid at 45 °C for 6 h to obtain formula 1. The compound (44.42 g) was shown in a yield of 81.2% and a purity of 99.7% by HPLC.
 | [target]
Primary target: MET | [Drug interactions]
Tepotinib is indicated for the treatment of adult patients with
metastatic non-small cell lung cancer (NSCLC) who have
mesenchymal-epithelial transition (_MET_) exon 14 skipping alterations. | [Metabolism]
Tepotinib is metabolized primarily by CYP3A4 and CYP2C8, with some
apparent contribution by unspecified UGT enzymes. The metabolite M506 is
the major circulating metabolite, comprising approximately 40.4% of
observed drug material in plasma, while the M668 glucuronide metabolite
has been observed in plasma at much lower quantities (~4% of an orally
administered dose). A total of 10 phase I and phase II metabolites have
been detected following tepotinib administration, most of which are
excreted in the feces. |
| Questions And Answer | Back Directory | [Binding Mode]
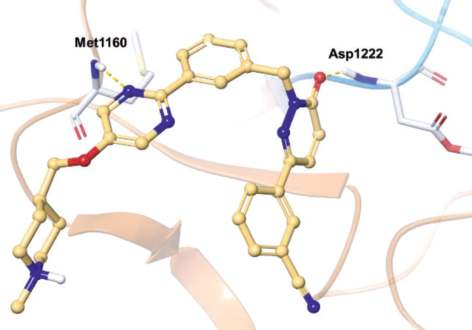
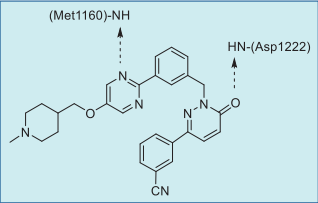
In the co-crystal structure of tepotinib bound to
MET (Fig. 1),
the pyrimidine nitrogen hydrogen
bonds with the amide NH group of Met1160 (the third
hinge residue) and the pyridazinone oxygen
hydrogen bonds to the amide NH group of DFG-Asp1222 (Fig. 2). The inhibitor binds to a DFG-in and
αC-out inactive conformation as a type I? inhibitor.
  |
|
|