| Identification | Back Directory | [Name]
(S)-2-HYDROXYMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER | [CAS]
1030377-21-9 | [Synonyms]
(S)-1-Boc-2-(hyroxyMethyl)piperazine
(S)-N1-BOC-2-Hydroxymethyl-piperazine
(S)-1-N-BOC-2-HYDROXYMETHYLPIPERAZINE
(2S)-2-(Hydroxymethyl)piperazine, N1-BOC protected
(S)-tert-butyl 2-(hydroxyMethyl)piperazin-1-carboxylate
(S)-tert-butyl 2-(hydroxyMethyl)piperazine-1-carboxylate
tert-butyl (S)-2-(hydroxymethyl)piperazine-1-carboxylate
tert-butyl (2S)-2-(hydroxymethyl)piperazine-1-carboxylate
1,1-Dimethylethyl (2S)-2-(hydroxymethyl)-1-piperazinecarboxylate
(S)-2-HYDROXYMETHYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
(2S)-2-(hydroxymethyl)-1-Piperazinecarboxylic acid tert-butyl ester
1-PIPERAZINECARBOXYLIC ACID, 2-(HYDROXYMETHYL)-, 1,1-DIMETHYLETHYL ESTER, (2S)-
tert-Butyl (2S)-2-(hydroxymethyl)piperazine-1-carboxylate, (2S)-1-(tert-Butoxycarbonyl)-2-(hydroxymethyl)piperazine | [Molecular Formula]
C10H20N2O3 | [MDL Number]
MFCD07772093 | [MOL File]
1030377-21-9.mol | [Molecular Weight]
216.28 |
| Hazard Information | Back Directory | [Reactions]
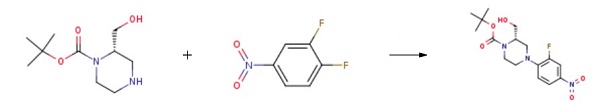
(S)-1-Boc-2-(Hydroxymethyl)piperazine reacts with 1,2-difluoro-4-nitrobenzene to synthesize tert-butyl (S)-4-(2-fluoro-4-nitrophenyl)-2-(hydroxymethyl)piperazine-1-carboxylate. This compound serves as a crucial intermediate in the preparation of the Wee-1 kinase activity inhibitor.
 | [Synthesis]
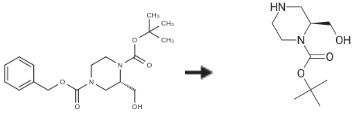
To a solution of (S)-4-benzyl 1-tert-butyl 2-(hydroxymethyl)piperazine-1,4-dicarboxylate (1.5 g, 4.28 mmol) in MeOH (20 mL), 10 % Pd/C (150 mg) was added. The mixture was stirred at room temperature for 3 hours under H2. Afterwards, the solid was removed by filtration and the filtrate was concentrated to yield (S)-1-Boc-2-(Hydroxymethyl)piperazine (850 mg, 3.93 mmol, 91.82 percent) as a white solid.
|
|
|