| Identification | Back Directory | [Name]
INCB28060 | [CAS]
1029712-80-8 | [Synonyms]
NC280
INC280
CS-192
INC28060
INCB28060
NVP-INC280
INCB028060
CapMatinib
BenzaMide HCl
Capmatinib base
INCB28060, INC280
INC280 Capmatinib
CAPMATINIB (INC280)
BenzaMide INCB28060
INC 280 (INCB28060)
INCB28060,BenzaMide
Capmatinib USP/EP/BP
INCB28060(Capmatinib)
CapMatinib (INCB28060)
INCB 28060; INCB-28060; INC280
CAPMATINIB (INCB28060);INCB-28060; INC-280
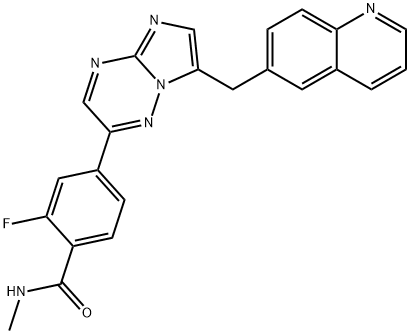
2-Fluoro-N-methyl-4-[7-(6-quinolinylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl]benzamide
2-Fluoro-N-Methyl-4-(7-quinolin-6-ylMethyl-iMidazo[1,2-b][1,2,4] triazin-2-yl)-benzaMide
BenzaMide, 2-fluoro-N-Methyl-4-[7-(6-quinolinylMethyl)iMidazo[1,2-b][1,2,4]triazin-2-yl]-
NC280 BenzaMide,2-fluoro-N-Methyl-4-[7-(6-quinolinylMethyl)iMidazo[1,2-b][1,2,4]triazin-2-yl]-
BenzaMide, 2-fluoro-N-Methyl-4-[7-(6-quinolinylMethyl)iMidazo[1,2-b][1,2,4]triazin-2-yl]-
INCB28060
INC28060(BenzaMide, 2-fluoro-N-Methyl-4-[7-(6-quinolinylMethyl)iMidazo[1,2-b][1,2,4]triazin-2-yl]-)
2-fluoro-n-methyl-4-[7-[(quinolin-6-yl)methyl]imidazo[1,2-b]-[1,2,4]triazin-2-yl]benzamide capmatinib
2-Fluoro-N-methyl-4-[7-[(quinolin-6-yl)methyl]imidazo[1,2-b]-[1,2,4]triazin-2-yl]benzamide Capmatinib (INCB28060) | [EINECS(EC#)]
813-241-9 | [Molecular Formula]
C23H17FN6O | [MDL Number]
MFCD18633285 | [MOL File]
1029712-80-8.mol | [Molecular Weight]
412.419 |
| Chemical Properties | Back Directory | [Melting point ]
>250°C (dec.) | [density ]
1.40 | [vapor pressure ]
0-0Pa at 20-25℃ | [storage temp. ]
-20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
13.82±0.46(Predicted) | [color ]
Pale Yellow to Light Yellow | [InChI]
InChI=1S/C23H17FN6O/c1-25-22(31)18-6-5-16(11-19(18)24)21-13-28-23-27-12-17(30(23)29-21)10-14-4-7-20-15(9-14)3-2-8-26-20/h2-9,11-13H,10H2,1H3,(H,25,31) | [InChIKey]
LIOLIMKSCNQPLV-UHFFFAOYSA-N | [SMILES]
C(NC)(=O)C1=CC=C(C2=NN3C(CC4=CC=C5C(=C4)C=CC=N5)=CN=C3N=C2)C=C1F | [LogP]
1.6-2.2 at 35℃ and pH7-9 |
| Questions And Answer | Back Directory | [MET small molecule inhibitor]
Capmatinib(Synonyms: INC280; INCB28060) is a competitive inhibitor with very potent and selective activity against MET compared to other kinases. It has been shown in vitro that cell lines made resistant to erlotinib, an EGFR inhibitor, could be resensitized after capmatinib treatment. Results from a phase Ib/II study of patients with EGFR-mutated, MET-dysregulated NSCLC have shown promising responses to a combination of capmatinib and gefitinib (EGFR TKI) following disease progression from an only EGFR TKI treatment regimen. Recommended phase II dose was determined to be capmatinib 400 mg twice/day and gefitinib 250 mg once/day. Most common adverse events were nausea, peripheral edema, decreased appetite, rash, and increased amylase and lipase levels. | [Originator]
Novartis
|
| Hazard Information | Back Directory | [Description]
Capmatinib is a competitive inhibitor with very potent and selective activity against MET compared to other kinases. | [Uses]
2-Fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzamide is used in the preparation of a combination formulation with allosteric SHP2 inhibitor TNO155. Used in treatment of non-small cell lung cancer with specific mutations (those that lead to mesenchymal-epithelial transition or MET exon 14 skipping). | [Brand name]
Tabrecta | [General Description]
Class: receptor tyrosine kinase;
Treatment: NSCLC with METex14;
Other name: INCB28060;
Oral bioavailability >70%;
Elimination half-life = 7.8 h;
Protein binding = 96% | [Side effects]
Most common adverse events were nausea, peripheral edema, decreased appetite, rash, and increased amylase and lipase levels. | [in vitro]
It has been shown in vitro that cell lines made resistant to erlotinib, an EGFR inhibitor, could be resensitized after capmatinib treatment. Results from a phase Ib/II study of patients with EGFR-mutated, MET-dysregulated NSCLC have shown promising responses to a combination of capmatinib and gefitinib (EGFR TKI) following disease progression from an only EGFR TKI treatment regimen. Recommended phase II dose was determined to be capmatinib 400 mg twice/day and gefitinib 250 mg once/day. | [target]
c-MET | [Dosage]
The recommended capmatinib dose is 400 mg orally twice daily with or without food. | [References]
1. liu x, wang q, yang g, et al. a novel kinase inhibitor, incb28060, blocks c-met-dependent signaling, neoplastic activities, and cross-talk with egfr and her-3. clinical cancer research : an official journal of the american association for cancer research. 2011;17(22):7127-7138. |
|
|