| Identification | Back Directory | [Name]
Uranium tetrafluoride | [CAS]
10049-14-6 | [Synonyms]
Uranium tetrafluoride
Uranium(IV) fluoride.
Uranium fluoride(UF4)
Uran(IV) tetrafluoride | [EINECS(EC#)]
233-170-1 | [Molecular Formula]
F4U | [MDL Number]
MFCD00661066 | [MOL File]
10049-14-6.mol | [Molecular Weight]
314.023 |
| Chemical Properties | Back Directory | [Melting point ]
>1100° | [Boiling point ]
1446.94°C (estimate) | [density ]
6.700 | [solubility ]
soluble in concentrated acid solutions, alkaline solutions | [form ]
green monoclinic crystals | [color ]
green monoclinic crystals, crystalline | [Water Solubility ]
insoluble H2O; soluble conc acids, alkalies, but decomposes [MER06] | [EPA Substance Registry System]
Uranium tetrafluoride (10049-14-6) |
| Hazard Information | Back Directory | [Chemical Properties]
Green, nonvolatile, crystalline powder.
Insoluble in water. | [Uses]
Intermediate in preparation of uranium metal.
See uranium hexafluoride. | [Hazard]
Highly corrosive, radioactive poison. | [Description]
Uranium tetrafluoride, with the molecular formula of UF4, is a green, nonvolatile, crystalline powder that is insoluble in water. It is highly corrosive and is also a radioactive poison. | [Preparation]
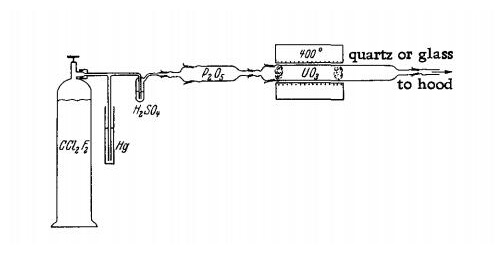
Dichlorodifluoromethane (Freon 12) is passed through a Hg pressure release valve, a bubble counter and aPsO5 tube into a glass or quartz reaction tube (diameter 2.5 cm, length 40 cm.) The reaction tube is inserted into a short electric furnace which can be heated to a temperature of 400℃. Powdered UO3 is placed in the reaction tube between glass-wool plugs. The escaping gases are led to the hood.
At the beginning, dry oxygen is passed through the apparatus for one hour, while the furnace is heated to 400℃. The oxygen flow is then replaced with CF2Cl2, which is introduced at a rate of one liter per hour. The reaction starts as soon as the temperature reaches 400°C. The progress of the reaction can be followed as the color of the product changes to green.
On completion of the reaction, the product is cooled in a stream of CF2Cl2; very pure UF4 is obtained. The yield is almost quantitative.
|
|
|