| Identification | Back Directory | [Name]
Sodium picosulfate | [CAS]
10040-45-6 | [Synonyms]
LA 391
C13072
DA 1773
DA-1773
Evacuol
Picolax
Laxoberal
sodium picosulfate
Picosulfate Sodium
PICOSULPHATE SODIUM
Bisacodyl IMpurity D
Bisacodyl Impurity 4
Sodium picosulfate CRS
SodiuM Picosulphate USP
Sodium Picosulfate (50 mg)
Sodium Picosulfate Hydrate
Sodium picosulfate USP/EP/BP
Sodium Picosulfate (1614578)
Sodium Picosulfate n -Hydrate
Ergotamine Tartrate Impurity 6
Picosulfate for system suitability CRS
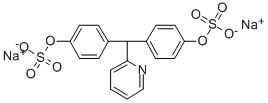
2-[Bis[p-(sodiooxysulfonyloxy)phenyl]methyl]pyridine
4,4'-(pyridin-2-ylmethylene)bisphenyl bis (sodiumsulphate)
Sodium (pyridin-2-ylmethylene)bis(4,1-phenylene) bis(sulfate)
Disodium bis[4-[(hydroxysulfonyl)oxy]phenyl](2-pyridyl)methane
4,4'-(2-Pyridinylmethylene)bis(phenol)bis(sulfuric acid sodium) salt
4 4'-(2-PYRIDINYLMETHYLENE) BISPHENOL BI S(HYDROGEN SULFATE )(ESTER) DISODIUM SALT
4,4`-(pryidine-2-ylmethylene)bisphenyl bis (sodium sulphate), (SODIUM PICOSULFATE)
Phenol, 4,4'-(2-pyridinylmethylene)bis-, 1,1'-bis(hydrogen sulfate), sodium salt (1:2)
Sodium PicosulfateQ: What is
Sodium Picosulfate Q: What is the CAS Number of
Sodium Picosulfate Q: What is the storage condition of
Sodium Picosulfate Q: What are the applications of
Sodium Picosulfate
Picosulfate-d13 SodiumQ: What is
Picosulfate-d13 Sodium Q: What is the CAS Number of
Picosulfate-d13 Sodium Q: What is the storage condition of
Picosulfate-d13 Sodium Q: What are the applications of
Picosulfate-d13 Sodium | [EINECS(EC#)]
233-120-9 | [Molecular Formula]
C18H13NO8S2.2Na | [MDL Number]
MFCD00867640 | [MOL File]
10040-45-6.mol | [Molecular Weight]
481.41 |
| Chemical Properties | Back Directory | [Melting point ]
283-286°C | [storage temp. ]
Hygroscopic, Refrigerator, Under Inert Atmosphere | [solubility ]
Freely soluble in water, slightly soluble in ethanol 96 per cent. | [form ]
neat | [color ]
White to Off-White | [Stability:]
Hygroscopic | [InChIKey]
GOZDTZWAMGHLDY-UHFFFAOYSA-L | [SMILES]
C(C1N=CC=CC=1)(C1=CC=C(OS([O-])(=O)=O)C=C1)C1C=CC(OS([O-])(=O)=O)=CC=1.[Na+].[Na+] |
| Safety Data | Back Directory | [HS Code ]
2933399090 | [Toxicity]
mouse,LD50,intraperitoneal,2900mg/kg (2900mg/kg),LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATIONBEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD,Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 8, Pg. 341, 1977. |
| Hazard Information | Back Directory | [Chemical Properties]
Off-White Solid | [Originator]
Guttalax, De Angelini, Italy ,1967 | [Uses]
analgesic | [Uses]
Sodium Picosulfate inhibits absorption of water and electrolytes, and increases their secretion | [Uses]
Sodium picosulfate is a stimulant laxative related to bis-acedyl; used for the treatment of constipation and for evaluation of the colon by stimulation bowel movements following hydrolysis by colon bacteria. | [Definition]
ChEBI: Sodium picosulfate is an aryl sulfate and a member of pyridines. | [Manufacturing Process]
Preparation of 3,3'-Dichloro-4,4'-Dioxy-Diphenyl-(2-Pyridyl)-Methane: 75 g (0.7 mol) of 2-pyridinaldehyde are dropped during about 1 hour to a homogeneous mixture [obtained between 0° and 10°C from 107 ml of concentrated sulfuric acid and 292.9 g (2.28 mols) of 2-chlorophenol], maintaining the temperature between 0° and 5°C. The mixture is stirred for ? hour at this temperature, which is then allowed to rise spontaneously, taking care not to exceed 30°C. After stirring for 1? hours, the mixture is maintained overnight at room temperature, then it is dissolved, with external cooling, with a 10% sodium hydroxide solution, filtered with charcoal and neutralized with 5% hydrochloric acid. The precipitate obtained, consisting of crude product, filtered, washed with water, dried, triturated with ether and dried again, weighs 211 g.
The isomer 2,4'-dioxy-3,3'-dichloro-diphenyl-(2-pyridyl)-methane is removed by thoroughly washing with 430 ml of 95°C boiling alcohol, obtaining 167 g of isomer-free product (yield 69%). The 3,3'-dichloro-4,4'-dioxy-diphenyl-(2pyridyl)-methane is a white solid, crystallizing from 95% alcohol; MP 212° to 215°C.
Preparation of 4,4'-Dioxy-Diphenyl-(2-Pyridyl)-Methane: 100 g of 3,3'dichloro-4,4'-dioxydiphenyl-(2-pyridyl)-methane, obtained as above described, are dissolved in 660 ml of 10% sodium hydroxide and 49 g of Raney-nickel alloy are added to the solution with vigorous stirring, at room temperature and during 4 hours. The mixture is stirred overnight at room temperature, then it is filtered and brought to pH 5 with 10% acetic acid. The precipitate obtained, filtered, washed and dried is then dissolved in 1,500 ml of 95°C boiling alcohol to eliminate the insoluble salts. The residue obtained after the evaporation of the alcoholic solution weighs 74 g (yield 92%). The yield in respect to 2-pyridinaldehyde is 63.5%. The compound is a white solid, crystallizing from 95% alcohol; MP 248° to 250.5°C, according to US Patent 3,558,643.
Preparation of Disodium 4,4'-Disulfoxy-Diphenyl-(2-Pyridyl)-Methane: In ? hour, 102 g chlorosulfonic acid are added to a solution of 100 g 4,4'dihydroxydiphenyl-(2-pyridyl)methane in 750 ml of anhydrous pyridine, the temperature being maintained at between 0° and 5°C. Towards the end of the addition of acid, a precipitate is formed which is slowly redissolved during subsequent agitation.
Upon completion of the addition, the mixture is agitated for 7 hours at ambient temperature. The solution is then poured into 3 liters of water/ice obtaining a clear solution of dark yellow color which is rendered alkaline upon phenolphthalein with 30% NaOH and extracted with ethyl ether to eliminate the majority of the pyridine. The mixture is filtered with active charcoal, the pH adjusted to 8 with hydrochloric acid 1:1 and extracted with chloroform to remove the 4,4'-dihydroxydiphenyl-(2-pyridyl)-methane which has not reacted.
The aqueous solution is then concentrated to dryness at an outside temperature of 40° to 45°C and at low pressure. The residue, obtained by drying in a vacuum at 40° to 45°C is triturated in a mortar with ethyl ether and, after filtration, is extracted with 3,400 ml boiling absolute ethanol. The ethanol extract is separated from the undissolved part by filtration, cooled and the product which crystallizes by cooling is filtered and dried at 40°C in a vacuum. In that manner the disodium (4,4'-disulfoxy-diphenyl)-(2pyridyl)methane bi-hydrate is obtained, which takes the form of a white solid, according to US Patent 3,528,986. | [Therapeutic Function]
Laxative | [Side effects]
Major minor side effects for Sodium Picosulfate:Diarrhoea, Abdominal pain, Nausea and vomiting, and Stomach cramps.
|
|
|